AliveCor, global leader in personal electrocardiogram (ECG) technology announces KardiaMobile to monitor and alert people experiencing irregular heart rhythm symptoms, 24 hours in advance
AliveCor, the global leader in FDA-cleared personal electrocardiogram (ECG) technology, today announced that the National Institute for Health and Care Excellence (NICE) has issued Medical Technologies Guidance (MTG) recommending KardiaMobile as an option for detecting atrial fibrillation
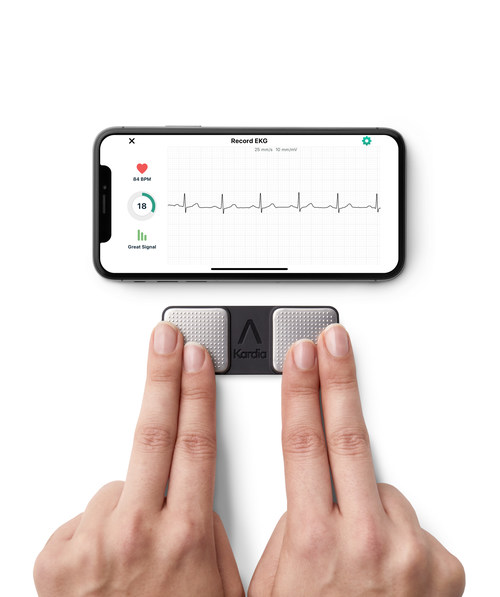
AliveCor, the global leader in FDA-cleared personal electrocardiogram (ECG) technology, today announced that the National Institute for Health and Care Excellence (NICE) has issued Medical Technologies Guidance (MTG) recommending KardiaMobile as an option for detecting atrial fibrillation (AF) for people with suspected paroxysmal AF, who present with symptoms such as palpitations and are referred for ambulatory ECG monitoring by a clinician. KardiaMobile is the first personal ECG to be recommended by NICE for use within the National Health Service (NHS) in England and Wales. KardiaMobile would be prescribed by a healthcare professional for people experiencing arrhythmia (irregular heart rhythm) symptoms more than 24 hours apart. The instructions for use state that all interpretations of ECG recordings are reviewed by a healthcare professional and used to support clinical decision making.
AF, the most common form of arrhythmia is a leading cause of AF-related stroke. Almost half of the 1.3 million people in the UK living with AF are undiagnosed, leaving them particularly at risk of suffering the life-changing, and often devastating, effects of this serious form of stroke.5 Stroke detection and prevention is key to the NHS Long Term Plan, in efforts to save thousands of lives and reduce the staggering costs of disease – approximately £3 billion per year direct costs to the NHS, with further £4 billion to the UK economy in lost productivity, disability and informal care.
“AF diagnosis rates across the UK pre-pandemic were already too low. With difficulties in accessing in-person care and increased waiting times, concern is that diagnosis rates have fallen further, leaving thousands of people at risk of life-threatening cardiovascular complications, such as an AF-related stroke” said Professor Matt Reed, RCEM Professor, Consultant and NRS Fellow in Emergency Medicine, NHS Lothian. “With the NHS over-stretched, it is encouraging that NICE has recognised the value of utilising smart technology to support clinicians. Today’s recommendation of KardiaMobile – a clinically-validated digital tool to allow people to monitor their heart rhythm at home, avoiding the need for hospital appointments – is a great step forward for cardiac services.”
“Many people experience various symptoms but do not realise it may be a heart rhythm disorder such as AF. If medical attention is not sought it leaves them exposed to a much higher risk of AF-related stroke. KardiaMobile can be used to monitor a person’s heart rhythm at any time, regardless of whether or not people show signs or symptoms of AF. This ultimately means AF can be detected faster, leading to a quicker diagnosis and therefore more lives being protected against AF-related stroke and consequences of suffering with AF,” said Mrs Trudie Lobban MBE, Founder and Trustee of Arrhythmia Alliance & AF Association.
KardiaMobile is the world’s most clinically-validated personal ECG. Unlike traditional ECGs, KardiaMobile provides a compact, patchless and wireless solution that can be used at any time and anywhere. The user starts a 30-second ECG recording on their smartphone via the Kardia app – by placing two fingers from each hand on each of the two top electrodes – enabling the patient to remotely capture a medical-grade recording of their heart activity. KardiaMobile provides instant detection of AF, bradycardia and tachycardia, which are leading indicators of cardiovascular disease. KardiaMobile can also detect normal heart rhythm, offering users peace of mind.
Commenting on the announcement, Priya Abani, CEO, AliveCor said, “AliveCor is proud to be able to offer the only NICE-recommended personal ECG to support remote cardiac care services for patients not in front of their cardiologist. Today’s recommendation not only highlights the clinical superiority of KardiaMobile against the current standard of care, but also its position as a more cost-effective solution, therefore warranting its value as a clinical tool to support rapid diagnosis of AF.”
NICE’s recommendation of KardiaMobile as a clinically and cost-effective medical technology is supported by evidence from 27 different studies, including five randomised controlled trials. Data submitted to NICE, showed KardiaMobile was five times more efficacious at detecting heart rhythm problems than standard tests alone, with greater usability.
NICE’s MTG evaluates new, innovative medical devices and diagnostics. It assesses medical technologies that deliver treatment, give greater independence to patients and detect or monitor medical conditions.
About AliveCor
AliveCor, Inc. is transforming cardiological care using deep learning. The FDA-cleared KardiaMobile device is the most clinically-validated personal ECG solution in the world. KardiaMobile 6L provides instant detection of Atrial Fibrillation, Bradycardia, Tachycardia, Sinus Rhythm with Supraventricular Ectopy, Sinus Rhythm with Premature Ventricular Contractions, Sinus Rhythm with Wide QRS and Normal Sinus Rhythm in an ECG. Kardia is the first AI-enabled platform to aid patients and clinicians in the early detection of atrial fibrillation, the most common arrhythmia and one associated with a highly-elevated risk of stroke. AliveCor’s enterprise platform allows third party providers to manage their patients’ and customers’ heart conditions simply using state-of-the-art tools that provide easy front-end and back-end integration to AliveCor technologies. AliveCor protects its customers with stringent data security and compliance practices, achieving HIPAA compliance and SOC2 Type 1 and Type 2 attestations. AliveCor is a privately-held company headquartered in Mountain View, Calif. “Consumer” or “Personal” ECGs are ECG devices available for direct sale to consumers.






