HMD Scaling up Production to 1 Billion Auto Disable Immunization Syringes to help in Covid-19 Vaccination
· HMD has received order from UNICEF to increase its supply of immunisation AD syringes to the organisation to around 300 million to build up a stockpile of around 140 million syringes for Covid-19 by the
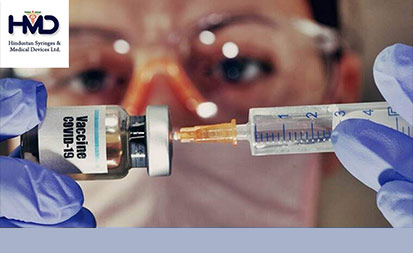
· HMD has received order from UNICEF to increase its supply of immunisation AD syringes to the organisation to around 300 million to build up a stockpile of around 140 million syringes for Covid-19 by the end of the year – Mr. Rajiv Nath, Managing Director of Hindustan Syringes & Medical Devices Ltd.
Hindustan Syringes & Medical Devices Ltd (HMD), one of the largest manufacturers of Disposable Syringes in the World and the largest for Auto Disable syringes with annual capacity of around 700 million auto-disable syringes for vaccination is scaling up production to a billion in the first half of 2021 as India gets ready for COVID-19 Vaccine.
“We have received orders from UNICEF to increase our supply of immunization AD syringes to the organisation to around 300 million to build up a stockpile of around 140 million syringes for Covid-19 by the end of the year” said Mr. Rajiv Nath, Managing Director of Hindustan Syringes & Medical Devices Ltd.
“We are waiting on the Indian government to start creating a stockpile of syringes as being done by other Countries. Should the government need 100 million auto-disable syringes for Covid-19 vaccines by the end of this year, we can easily offer them to lift the outstanding orders placed with us. We have nearly 50 million in stock that the government has not timely lifted as standard immunisation injection campaigns had been suspended at onset of Covid. Though the campaigns have been restarted recently, the pace is still slow,” added Mr. Rajiv Nath.
As the race for a ‘safe and effective’ vaccine against coronavirus infection is on the horizon, Pune based drugmaker Serum Institute of India is all set to start the advance clinical trial of University of Oxford vaccine while continuing to work alongside on 4 more vaccines candidates. In addition, Covaxin, India’s first coronavirus vaccine has been developed by Bharat Biotech, Indian Council of Medical Research (ICMR) and National Institute of Virology (NIV). The clinical trial to verify the safety of the potential COVID-19 vaccine started about two weeks back. If successful, it may be available by end of the year. Zydus is the 3rd of the 7 vaccine Manufacturers in the running.
“The outbreak of COVID-19 has taught the importance of infection prevention practices like hand-hygiene to all. The focus has shifted to single use disposable consumables from reuse consumables and especially a change has been seen in higher deployment of auto-disable syringes even for curative injections. WHO and. UNICEF also recommend that auto–disable syringes be used for administering vaccines— particularly in mass immunization programs.Moreover, WHO has suggested the use of auto-disable syringes to collect blood samples of Covid 19 patients, which in turn, helps to avoid the transmission of disease through healthcare equipment.
At the same time, HMD, which owns DispoVan and Kojak brands of syringes fears that while it’s an opportunity it also makes them nervous.“In past we did gear up to produce these as the largest manufacturer in world for AD Syringes only to find Indian Govt. buying from China at L1 rates and expected us to match these non remunerative rates. This product is like making traffic red-light, only Govt. buys red lights for traffic management and if they don’t, you have no market in private sector.”, explained Mr. Rajiv Nath.
“Over the years we moved over to more dependable international buyers who don’t have the silly criteria of L1 prices to be matched and have while expecting competitive pricing do give weightage to good quality and performance capabilities too.”, added Mr. Nath.
Mr. Rajiv Nath also raised concerns of the poor status of PPE , Masks & Ventilator mfrs who are saddled with surplus inventory & excess capacities after the supply chain mismatch crisis got over in June 2020.“So while we are expanding as it’s our national duty we do wonder after crisis is over about these under utilized investments- unlike USA who is supporting its manufacturers with Billions of dollars there is not yet an assurance to buy by Indian Govt. and orders placed in past are lying with us at our cost and not lifted nor paid for. This is Risky Business.” He added.
With over 9 plants, HMD has created a niche for their disposable syringe ―DISPOVAN which is today the most popular brand in syringe market in India with over 60% market share with Dispovan Needle and Disposable Insulin Syringes having over 70% Market Share and thereby displaced renowned MNC‘s – an inspirational case study for other Indian entrepreneurs.
HMD is one of the largest suppliers to UNICEF for Auto Disable Syringes for immunization and is the first Company in India to manufacture Auto Disable Syringes for Curative Segment.
HMD is regularly supplying KOJAK Auto disable Syringes for immunization campaigns conducted by the Ministry of Health (Govt.of India) & UNICEF & few Third World Countries where reuse of injections risky practices continue to exist. For the Curative Sector, HMD is supplying its Auto Disable Syringes for re- use prevention to various State Governments like-Punjab, Andhra Pradesh, Bihar, Madhya Pradesh etc. apart from exporting into many Countries in Africa.
As India gets ready for COVID-19 vaccine, the Govt. should be well equipped with secured stock of syringes in advance to administer a vaccine when it is approved and ready. At least 60 to 70% of 1.3 Billion people in India and 7.8 Billion people Worldwide will need syringes for Covid-19 vaccine.
HMD’s commitment to produce billions of immunisation AD syringes for the global COVID-19 vaccination reinforces the company’s vision to contribute to better healthcare in the country and internationally in areas of injection safety, patients safety, affordable access and stand with the country to fight the deadly virus.






