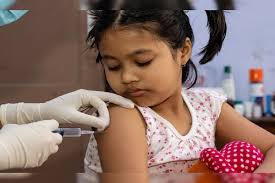
COVAXINÒ is the one of the first COVID-19 vaccines in the world to generate data in 2-18 year age group. Phase II/III, Open-Label, Multi-centre Study was conducted to evaluate the Safety, Reactogenicity, and Immunogenicity of the Whole-Virion Inactivated SARS-CoV-2 Vaccine (COVAXIN®) in healthy children
POST TAGS:
Bharat BiotechBIOPOLIOCentral Drugs Standard Control OrganisationChairman and Managing DirectorChikungunyaCOVAXIN® for Children: Study demonstrates robust safety and immunogenicity in 2-18 Volunteers.covid 19 vaccinesDr. Krishna Ellahealthy childrenHyderabad.immunogenicity in childrenIndiaInternational LimitedOmicron variantROTAVACsaidWHOWorld Health Organization