ADMI welcomes the government's decision to maintain stringent clinical trial requirements for In-Vitro Diagnostics (IVDs), reinforcing the commitment to safety and efficacy in India’s healthcare sector. By refusing to waive clinical trials for IVDs approved in other developed countries, the government ensures that these devices
~The signing of this MoU underscores a long-term commitment to addressing India’s growing respiratory care needs and sets the stage for transformative improvements in the fieAerogen, an Ireland-based global leader in acute-care aerosol drug delivery, and Indian Association of Respiratory Care (IARC) signed a MoU
Sharp Sight Eye Hospitals, a leading name in eye care, has successfully launched its latest campaign promoting the importance of regular eye checkups. The campaign, featuring renowned actor Shishir Sharma as its ambassador, has already made a significant impact, resulting in a noticeable increase in
Gleneagles Hospital Parel embraced the vibrant festival of Navratri with full enthusiasm, as employees donned traditional attire in the designated colors of the festival. The hospital premises were filled with a festive spirit, showcasing the rich cultural diversity of India. Each color worn during Navratri
The Indian Stroke Association (ISA) has launched MISSION BRAIN ATTACK, an initiative aimed at enhancing the awareness, education, and training of healthcare professionals in stroke prevention, immediate treatment, and rehabilitation. The campaign “Each One Teach One” addresses the alarming rise in stroke cases across India,
Ahead of World Mental Health day, Renowned doctor, Geeta Shroff organised a public awareness session, where they announced the launch of their dedicated women centric mental health clinic Lighthouse Counselling Centre, specially designed to support women’s mental well-being and offer specialized medical services.Present on the
Dr. Chethana D, Consultant – Rheumatology, Aster CMI Hospital, BangalorePsoriatic ArthritisPsoriatic arthritis (PsA) is a chronic inflammatory condition that affects both the skin and joints, emerging in individuals with psoriasis, a skin disorder characterized by red, scaly patches. This autoimmune disease can lead to significant
Sankara Eye Foundation India announced the launch of India’s first state-of-the-art AI voice-based feedback system named SAHAI. As a pioneering technology that is set to revolutionize the patient feedback-gathering process at large, SAHAI leverages the latest Large Language Models (LLMs) for absolutely voice-based interaction in
"None can destroy iron, but its own rust can! Likewise, none can destroy a person, but their own mindset can."These profound words by Ratan Naval Tata resonate deeply with his extraordinary life and legacy. As the world mourns the loss of this towering figure in
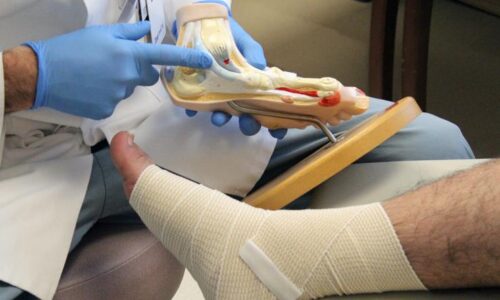
A team of skilled surgeons and doctors at Trilife Hospital successfully salvaged the left leg of a 57-year-old woman who suffered a severe degloving injury after her feet were run over by a bus. The patient, a resident of Assam, was visiting her daughter who