Black fungus drug allocation has stopped, Centre tells HC
While taking stock of various Covid-19 related aspects and monitoring the response of various authorities, the HC had also examined the shortage of medicines required to treat black fungus.New Delhi: The Centre on Wednesday informed
While taking stock of various Covid-19 related aspects and monitoring the response of various authorities, the HC had also examined the shortage of medicines required to treat black fungus.
New Delhi: The Centre on Wednesday informed the Delhi high court that it has stopped allocating the key drug needed to treat mucormycosis (black fungus) patients, since the drug’s availability has increased while cases have dipped.
While taking stock of various Covid-19 related aspects and monitoring the response of various authorities, the HC had also examined the shortage of medicines required to treat black fungus. In a status report, the central government told the HC that there has been a drop in mucormycosis cases, which were at a peak of 28,475 on June 27. As per the latest figures, the cases were 18,833 on July 30.
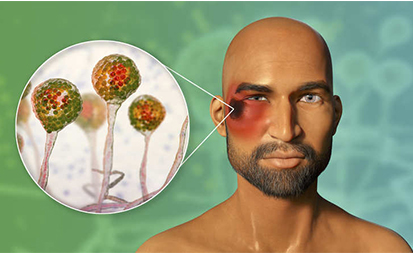
According to the Union health ministry, the infection is contracted by coming in contact with fungal spores in the environment. It can also develop on the skin after the fungus enters the skin through a cut, scrape, burn, or another type of skin trauma.
During the hearing, the Centre told the HC that the manufacturing capacity of Liposomal Amphotericin B, which is used for treating mucormycosis — mainly infecting Covid-recovered patients — and was in shortage earlier, has been augmented.
Hence, the centralised allocation of the medicine to the states has been discontinued and now they will have to procure the drug directly from the manufacturers, the Centre’s counsel told the HC.






