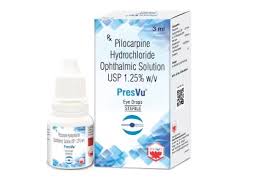
Mumbai-based ENTOD Pharmaceuticals has taken steps to address concerns raised by the Drug Controller General of India (DCGI) regarding its newly approved PresVu eye drops, intended for the treatment of presbyopia in adults. The leadership team of ENTOD met with the DCGI earlier this week,
Entod Pharmaceuticals has announced that it has received final approval from the Drug Controller General of India (DCGI) for its groundbreaking PresVu eye drops. This approval, disclosed during a press conference held today in New Delhi, follows a favorable recommendation by the Subject Expert Committee
According to an official statement on Friday, India has granted homegrown drugmaker Biological E permission to begin midtage studies of its COVID-19 vaccine in children and adolescents. The Drugs Controller General of India (DCGI) gave the approval to the Hyderabad-based pharmaceutical company on Sept. 1, the statement said. The
According to the sources, The DCGI (The Drugs Controller General of India) granted permission to Mukesh Ambani-owned Reliance Life Sciences to conduct its COVID-19 vaccine trial on friday with certain conditions. The safety, tolerability and immunogenicity of the SARS-CoV-2 recombinant protein subunit vaccine in healthy
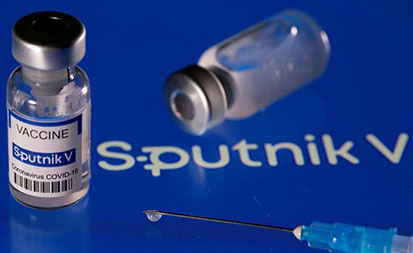
India’s drug regulator has refused to grant emergency-use authorisation to single-dose COVID-19 vaccine Sputnik-Light, while ruling out the need for the conduct of the phase-3 trial of the Russian vaccine in the country.According to the recommendations of the Subject Expert Committee (SEC) meeting held on
Delhi is likely to receive the first consignment of Sputnik V, the Russian COVID-19 vaccine, in June, Delhi Chief Minister Arvind Kejriwal said on Monday, while stressing that vaccination is key to the fight against coronavirus. The chief minister also said the city currently has