Healthium Medtech, one of India’s leading medical devices companies, has announced the acquisition of a controlling stake in Paramount Surgimed, a well-known manufacturer of surgical consumables and sterile medical products. The strategic acquisition is expected to expand Healthium’s product portfolio, strengthen its surgical offerings, and
India is rapidly cementing its position as a global powerhouse in the medical devices industry, driven by robust policy support, rising manufacturing capabilities, and increasing global demand for high quality, affordable medtech solutions. With a combination of strong domestic reforms and strategic international partnerships, the
New Delhi, 06th Nov 2025: SS Innovations International, Inc. (Nasdaq: SSII), a developer of innovative surgical robotic technologies dedicated to making robotic surgery affordable and accessible to a global population, has once again redefined the boundaries of innovation with the unveiling of the world’s first
In a move aimed at supporting India’s fast-growing medical device industry, the Government of India has announced the alignment of packaging and labeling rules for medical devices, easing compliance requirements and promoting regulatory clarity for manufacturers.The Department of Consumer Affairs, in coordination with the Central
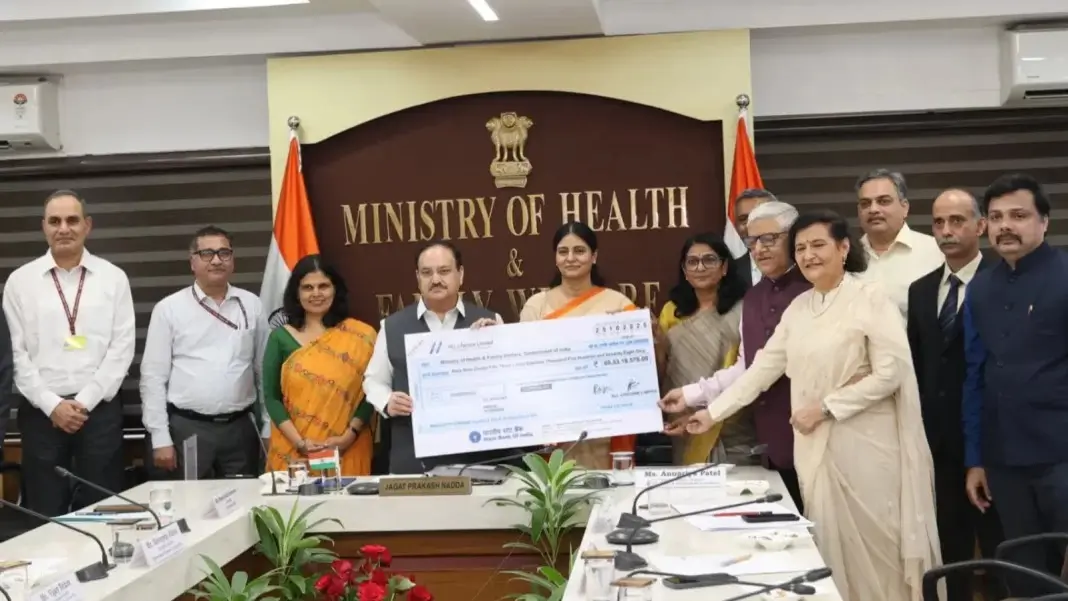
HLL Lifecare Limited (HLL), a Miniratna public sector enterprise under the Ministry of Health and Family Welfare (MoHFW), has paid a dividend of ₹69.53 crore to the Government of India for the financial year 2024–25, reaffirming its strong financial performance and contribution to India’s public
In a landmark move in the medical diagnostics sector, private equity firms Blackstone and TPG have announced plans to acquire Hologic, Inc., a leading medical technology company specializing in women's health diagnostics, for up to $18.3 billion, including debt. This deal represents the largest acquisition
In a significant step toward advancing medical research and innovation, the All India Institute of Medical Sciences (AIIMS), Guwahati, has inaugurated a dedicated Clinical Trial Unit (CTU) to facilitate high-quality clinical research, drug development, and evidence-based healthcare in the North-Eastern region of India.The newly inaugurated
New Delhi, 15th October 2025: Healthium Medtech, a global medtech company with a growing portfolio across surgical, post-surgical, advanced wound care, arthroscopy and infection prevention, has announced an exclusive partnership with US-based Tisgenx, Inc., the world’s leading manufacturer of bovine pericardial tissue patches. These patches,
MedGate Today, India’s top and one of the world’s foremost healthcare magazines, is proud to announce its ambitious global expansion plan. After the overwhelming success of its Middle East and Africa editions, with a strong base established through its Dubai office, MedGate Today is now
The Government of India has announced the launch of the PRIP (Promoting Research and Innovation in Pharma-MedTech) scheme, inviting proposals from industry players, startups, and research organizations for high-impact projects aimed at strengthening India’s pharmaceutical and medical technology ecosystem. The initiative is designed to accelerate