ENTOD Pharmaceuticals Engages with DCGI Over PresVu Eye Drops Following Suspension of Approval
Mumbai-based ENTOD Pharmaceuticals has taken steps to address concerns raised by the Drug Controller General of India (DCGI) regarding its newly approved PresVu eye drops, intended for the treatment of presbyopia in adults. The leadership
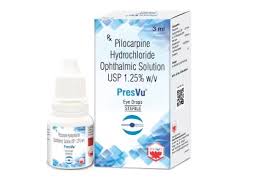
Mumbai-based ENTOD Pharmaceuticals has taken steps to address concerns raised by the Drug Controller General of India (DCGI) regarding its newly approved PresVu eye drops, intended for the treatment of presbyopia in adults. The leadership team of ENTOD met with the DCGI earlier this week, following the suspension of the product’s permission on September 10, 2024, due to safety concerns highlighted by health experts.
In a statement, ENTOD’s CEO, Nikkhil K. Masurkar, expressed understanding and respect for the DCGI’s decision. He emphasized the company’s commitment to transparency, stating, “We explained the PresVu situation to the DCGI and our sincere and honest intentions behind the media announcement.” Masurkar commended the swift action taken by the DCGI in the interest of public health.
The PresVu eye drops, which received approval on August 6, 2024, have been marketed as a therapeutic option for presbyopia, available only by prescription from registered medical practitioners. However, claims made during a recent press conference stirred controversy. ENTOD asserted that PresVu is the first eye drop in India designed to reduce dependence on reading glasses for those with presbyopia. This led to accusations from experts and health officials that the company was disseminating misleading information.
In response to the backlash, Masurkar clarified that the eye drops are not intended to replace reading glasses or non-invasive options. He noted that statements about the product’s efficacy, particularly regarding a 15-minute onset of action, were misinterpreted in some media reports, leading to confusion among the public.
The DCGI’s suspension followed these claims, which were viewed as potentially misleading. “Our press conference was done in good faith,” Masurkar stated, acknowledging the need for a clarification. He reiterated ENTOD’s dedication to regulatory compliance and the importance of maintaining trust with healthcare professionals and patients.
To reassure the DCGI, Masurkar provided a written undertaking to adhere strictly to the conditions of the approval and to make only sanctioned claims regarding PresVu. He also appealed for the reconsideration of the suspension.
Looking ahead, ENTOD plans to launch educational initiatives involving eye doctors and to train field staff and chemists to ensure awareness of the prescription-only status of PresVu eye drops. Vice President of Marketing, Mohammed Kamil Khan, welcomed the formation of clinical guidelines for the product by the apex body of ophthalmologists.
With a portfolio of over 150 ophthalmic formulations and a presence in 67 countries, ENTOD Pharmaceuticals continues to innovate in the field of eye care, currently focusing on treatments for myopia in children, glaucoma, corneal diseases, and retinal eye diseases. The company remains committed to maintaining its reputation as a trusted leader in ophthalmic solutions.